News
Shocking drug forgery: Indian firm denies it exported allergy-causing immunoglobulin vials
View(s):Health Ministry makes Rs. 40 million part payment to a supplier, without verifying from where or how the injections came to Sri Lanka
Medical community expresses serious concern over life-threatening corruption in the health sector
By Namini Wijedasa
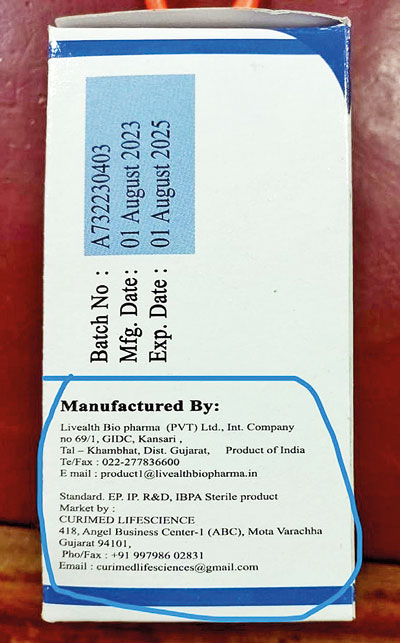
The packaging mentions an address in Gujarat with a Mumbai telephone number. It contains no licence number or any other details mandatory for the export of drugs
It was only when adverse reactions were recorded in Colombo, Kandy and Matale that doctors raised concerns about the human immunoglobulin 5-6g vials that had been distributed to government hospitals for intravenous use between July and September this year by the Medical Supplies Division (MSD).
The Sunday Times broke the story on October 1, 2023. A day later, Health Minister Keheliya Rambukwella said three separate complaints were lodged with the Criminal Investigation Department (CID), alleging that this immunoglobulin had been procured and cleared on the basis of a forged waiver of registration purporting to be from the National Medicines Regulatory Authority (NMRA) CEO. This is yet to be proven through an inquiry, either internal or external.
Had the allergies not drawn attention to this questionable tender, the full shipment of 22,500 vials is likely to have been bought at a cost of over Rs. 942 million.
Where did it come from?
Senior officials still do not know from where these immunoglobulin vials originated or who produced their contents. One even claimed (on condition of anonymity) that they could have been smuggled in on personal luggage, thereby compromising the cold chain and causing adverse reactions in patients. Or, he maintained, the local supplier—named the Seeduwa-based Isloez Biotech PharmaAG Ltd.—might have manufactured or compounded the drug here.
Health Ministry, MSD and NMRA documents state that the immunoglobulin was manufactured by an Indian company based in Gujarat. This is now established to be false.
On official documents in Sri Lanka, the Indian entity’s name is spelt in different ways, including ‘Livealth Bio Pharmaceuticals Pvt Ltd’ and ‘Livealth Biopharmas (Pvt) Ltd’. The problematic immunoglobulin vials were in boxes that said ‘Livealth Bio pharma [sic] (PVT) Ltd’.
The Sunday Times contacted the company. A representative categorically denied any connection with this order, saying Livealth Biopharma Pvt Ltd has not produced and is not even licensed to manufacture human immunoglobulin.
Categorical denial
“Please note that these products are neither manufactured nor supplied from our end,” he reiterated. “The best part is we have never manufactured, supplied or exported these products to any party to date.” Human immunoglobulin is not even on the list of its advertised items. And the drug is made by just three to four companies in India, he said.
On October 3, two days after our report, the MSD issued a circular ordering the human immunoglobulin IV 5-6g vials to be immediately withheld from use based on the recommendation of the NMRA’s Safety and Risk Evaluation Subcommittee. SAFRESC had given the guidance on September 20, two weeks prior. Samples were instructed to be made available to the NMRA quality assurance lab for tests. It is not immediately known whether this has been done.
It was on the same date, October 3, that Livealth in India first learned about its reported association with immunoglobulin through an email from the NMRA’s regulatory pharmacist. On receipt of this communication, the company did a limited search and acquired three product images circulating in the pharmaceutical community in relation to “a consignment fraudulently forwarded to Sri Lanka under our name”.
It wrote back to the NMRA that all information on the product boxes, including company name, address, and email, was incorrect. “We believe this has been done intentionally to avoid being identified in case of such happenings,” the representative said. The packaging mentions an address in Gujarat with a Mumbai telephone number. It contains no licence number or any other details mandatory for the export of drugs. 
“We wonder what paperwork was submitted to legitimise export,” he said, adding that Indian Customs and Drug Regulatory Authority are “extremely vigilant and strict about minute details”. “The question in our minds is, was this even exported from India? Looking at this packaging, this consignment could not have been passed or allowed for export.”
The packaging also mentions another company named “Curimed Lifescience” with a Gujarat address. The Livealth spokesman said he couldn’t vouch for this entity with which Livealth had no association. But he pointed out that the telephone number on the packaging attributed to Curimed had 11 digits instead of the usual ten.
No response from NMRA
Livealth has asked NMRA to identify “the source of such fraudulent and unwarranted activities under our label”. For its own investigation, it has requested the full names of the Indian exporter and Sri Lankan importer, the Indian port of dispatch, and the Sri Lankan port of arrival.
“With the above information, once received from your end, we would like to officially file a complaint with the Indian Central FDA [Food and Drug Administration], State FDA, Zonal FDA and Indian Customs to ensure such intentional fraudulent companies are identified and brought to justice to save genuine exporters’ image and more importantly precious human lives,” Livealth notified NMRA.
NMRA had not responded to the Indian company at the time of going to press. But the regulator issued a statement this week regarding what it said was the import and distribution of human immunoglobulin by Isolez, the quality failure of the drug, and the submission of forged documents to gain Customs clearance for the consignment.
After adverse reactions were reported from the Matale District Hospital and the National Hospital of Sri Lanka, the NMRA inquired into whether the drug was brought on a waiver of registration (WoR) that it had issued, the statement said. But there was no record whatsoever in the NMRA of this medicine consignment or any evidence of approval being granted. The product was neither registered nor exempted from registration. The relevant documents, including the official seal of the NMRA CEO, were forgeries.
Isolez Chairman Hewage Sudath Janaka Fernando admitted that they received a part payment for the vials they had supplied so far. (The Health Minister told parliament that 3,085 of the requisitioned 22,500 vials were received.) Mr.Fernando protested that the product was withdrawn without a quality lab test. And that the NMRA’s SAFRESC had not been appointed in terms of the law. He also contested the authenticity of the adverse reaction reports filed by the Kandy and Matale hospitals.
Mr.Fernando requested the Sunday Times not to publish other responses he provided to our questions—such as who supplied the immunoglobulin or whether Isolez had an agreement with Livealth—saying he had answered on the basis of confidentiality.
Isolez is not a member of the Sri Lanka Chamber of Pharmaceutical Industry.
Dangerous situation
The risks related to the low standard of human immunoglobulin are considerable, medical specialists unanimously agreed. The product directly affects human lives—it could spread blood-borne diseases such as HIV and hepatitis B or C—and is globally manufactured under stringent, specific conditions that are highly regulated. It has to be under refrigeration and is usually stored in separately-managed facilities.
In Sri Lanka’s case, there is zero information from the NMRA or Health Ministry about the stocks that were distributed among patients or what was even administered to them. Medical experts pointed out that the situation was dangerous because immunoglobulin is extracted from large quantities of human blood from many contributors.
Although the NMRA and the Health Ministry absolve themselves of guilt by stating the stocks were brought on forged documents, the MSD made the payment and released the drugs to hospitals. Health Secretary Janaka Chandraguptha confirmed he was the head of the relevant tender board. His signature is on a letter accepting expressions of interest from Isolez under the Indian credit line. This document was not claimed to be forged.
Yesterday, Mr. Chandraguptha confirmed his role on the tender board and said Cabinet approval hadn’t been necessary for the immunoglobulin purchase. He said to call him back for further details and thereafter did not answer the phone. The purchase passed many gatekeepers, but the “forgery” story surfaced only after allergies were reported.
It is now critical to check whether the NMRA CEO’s alleged “forged seals” were used to buy other medications, medical professionals urged. All regulatory processes have broken down, as evidenced by this case. The issuance of indiscriminate WoRs by the NMRA on the Health Ministry’s instructions has opened the floodgates.
“When the regulatory system is weakened or abused, when the regulator is asleep, this is what happens,” a senior doctor concluded.
The best way to say that you found the home of your dreams is by finding it on Hitad.lk. We have listings for apartments for sale or rent in Sri Lanka, no matter what locale you're looking for! Whether you live in Colombo, Galle, Kandy, Matara, Jaffna and more - we've got them all!

