News
Spotlight on recent incidents & deaths in state health sector
View(s):- NMRA Chief answers Sunday Times queries in a no-holds-barred interview and requests setting up of accredited lab to check meds
By Kumudini Hettiarachchi
In the wake of several incidents and deaths being reported from some state hospitals in the country, the Sunday Times asked the drug watchdog, the National Medicines Regulatory Authority (NMRA) for specifics in each case.
This was as earlier in the week, the Health Ministry appointed a seven-member ‘Expert Committee’ to investigate the recent incidence of drug allergies and their after-effects.
At a meeting with NMRA Chairman Prof. S.D. Jayaratne and CEO Dr. Vijith Gunasekera on Thursday, these are the specifics gathered by the Sunday Times: 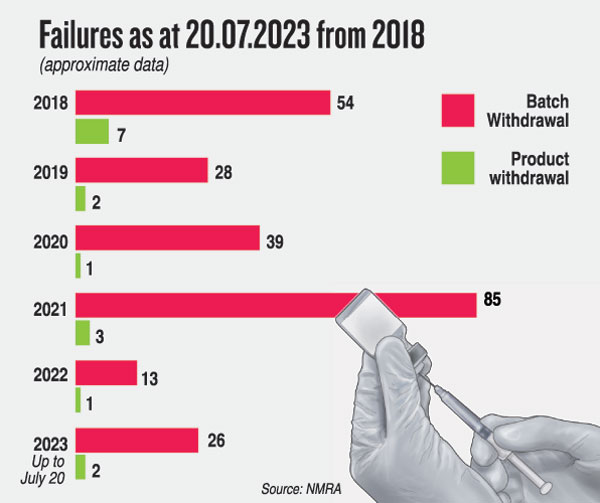
Death of a 21-year-old woman admitted to the Peradeniya Teaching Hospital with symptoms of food poisoning, administered the antibiotic Ceftriaxone. She had reportedly died of anaphylaxis (a serious life-threatening allergic reaction).
“The current batch of Ceftriaxone was brought under a Waiver of Registration (WOR) by a reputed pharmaceutical company,” said Prof. Jayaratne, explaining that it had earlier been registered for about 20 years without any issues. This batch and the batch at the Kandy National Hospital which had caused severe adverse events in three patients, have been withdrawn. This antibiotic injection, however, had been used on 12 other patients at Peradeniya without any allergic reaction.
Looking at the death of a four-month-old baby at Kuliyapitiya, he said that the child had got the pentavalent vaccine the previous day at Hettipola and had been fine. If there was anaphylaxis it would have come on immediately.
Reiterating that people should not lose faith in life-saving vaccines, he said that investigations are on to ascertain the reasons for the child’s unfortunate death. From the vial, the child had been vaccinated, 20 other children too had got their shots without a problem. The pentavalent vaccine is administered under Sri Lanka’s important and very successful Expanded Programme on Immunization (EPI).
With regard to the death of an NMRA employee at the Panadura Hospital allegedly due to an infection after the insertion of a cannula, he said the person had sought treatment from a hospital in the south for viral fever and been later discharged. It had been there that the cannula was inserted and the site had been red and swollen. When he came to Panadura, where he was boarded, he had got himself admitted to the Panadura Hospital, once again with fever. By the time he went there, the infection had spread to the heart valves.
Referring to the incident where a 35-year-old woman who had undergone lens-change surgery at the National Eye Hospital of Sri Lanka died, Prof. Jayaratne said that preliminary investigations and the postmortem report had pointed to major co-morbidities including triple heart vessel disease.
Dealing with the two deaths – one of an expectant mother during a Caesarian-section and another of an elderly woman who was due to undergo a hernia operation – at the Peradeniya Hospital reportedly due to the spinal anaesthesia, bupivacaine, he said that this was brought under a WOR, once again by a reputed company. It too had registered this drug earlier but the registration had lapsed. This company had bid under the Indian Credit Line (ICL) for this tender. (See box on tender awarding)
In this no-holds-barred interview, Prof. Jayaratne said that any drug has two main components – the Active Pharmaceutical Ingredient/s (API) and the excipient, an inactive substance that serves as the vehicle or medium for the drug. In the case of bupivacaine, the assay showed that there was a very small problem with the glucose levels in the excipient. Even though, it could not have caused the deaths, the full batch has been withdrawn.
On the severe adverse reactions caused by propofol, an intravenous anaesthetic used for procedural sedation, the NMRA Chairman said it had been brought under a WOR, once again under the ICL. There had been an endotoxin contamination giving rise to a fever reaction. This batch of propofol has been withdrawn.
Regarding several people going blind at the National Eye Hospital, Colombo, and the Nuwara Eliya Hospital, after the use of the eye-drop Prednisolone Acetate Ophthalmic Suspension USP 10-PRED-S, a common steroid and anti-inflammatory medication, he said that it had been contaminated by the micro-bug Burkholderia cepacia (B. cepacia).
He added that this eye-drop is registered and has been used for eight years without an issue. It is imported from India, once again by a reputed pharma company. The contamination has compelled the withdrawal of the batch.
Conceding that the NMRA lacked the capacity to check samples of drugs, Prof. Jayaratne suggested the establishment of an accredited laboratory even with international collaboration. Then not only would the NMRA be able to check random samples of registered products but also those being brought under WORs, he said, adding that this would plug the loophole some may be using to pass substandard products into Sri Lanka.
Meanwhile, the Expert Committee chaired by the Director of the Medical Research Institute (MRI), Dr. Dedunu Dias, has been tasked with submitting its report in three weeks.
‘NMRA not part of tender awarding process’
“The NMRA is not involved in any drug tender awarding process or procurements. We are the regulator for registrations to ensure safety, efficacy and affordability,” stressed NMRA Chairman Prof. S.D. Jayaratne, pointing out the drug watchdog’s mandate under the NMRA Act. Prof. Jayaratne who took over the NMRA in July 2022, after a turbulent period for the watchdog with many people being moved out etc., explained, step-by-step, the process for drug purchases in the state health sector: The process starts with a hospital’s Therapeutic Committee sitting down each year to tot up the figures of the drugs they require for the following year. This list is then submitted to the Medical Supplies Division (MSD) of the Health Ministry. The MSD collects all the lists from the hospitals. Usually, the total requirement of drugs would be between 8,000-10,000 varieties and devices around 20,000. Of the drug requirement, around 15% is manufactured in Sri Lanka and the balance imported, mainly from India. At national level, the MSD-appointed Therapeutic Committee reviews all the hospital lists and makes the final list on the drug requirements for the state health sector. The State Pharmaceuticals Corporation (SPC) then floats the tenders for this requirement. This is also a long process as many checks have to be ticked off – whether the drug is registered with the NMRA; whether the company is reliable; whether it has been blacklisted etc. Prof. Jayaratne goes onto explain that with the COVID-19 pandemic, lockdowns and people not being able to access hospitals, the drug requirement had come down in 2021. In the next year (2022), the SPC had reduced the requirement going by COVID-19 time predictions, which may have contributed to the shortages being faced now, it is learnt. The pandemic was followed by the economic crisis and lack of dollars. Prof. Jayaratne says that in view of the financial crisis, companies faced a challenge in opening Letters of Credit (LCs) and thus there were problems in getting them to import drugs. When the usual tenders were called by the government for drug purchases, these companies did not bid and the government was compelled to get the requirements under “emergency” purchases through the Indian Credit Line and other credit agencies. This situation also led to drugs coming into the country in different forms to meet shortages, it is understood. He explains that as hospitals were hit by shortages, drugs came as donations (drugs which were registered but a majority which were not) or as ‘emergency requests’. Meanwhile, there was also a dangerous trend of people just bringing in drugs in their suitcases when returning from abroad. Citing Section 109 of the NMRA Act with regard to “emergency” requests, he says: “The Authority may grant permission in special circumstances such as to save a life, to control an outbreak of an infection or an epidemic or any other national emergency or for national security to import and supply a particular medicine, medical device or borderline product in specified quantities. “Such permission may be granted:— (a) on a request made by the Ministry of Health; or (b) on a request made by an individual or an organization recommended by the Ministry of Health. “The importer shall be responsible for the accountability and management of the medicine, medical device or borderline product imported under this section.” When the Health Ministry requests such emergency purchases, according to the NMRA Chief, there is no time for a full evaluation before registration as it takes much time. Then such requests fall under Waivers of Registration (WORs). For such WORs, there is a quick check by the NMRA of the following documents. The documents are: Whether the manufacturer is registered with the drug regulator in the country of manufacture Whether there is a ‘Certificate of Analysis’ for the manufacturing process Whether there is GMP (Good Manufacturing Process) Certification which is usually issued by the country/state regulator where the drug is manufactured Whether the manufacturers export or distribute the drug in their own countries Whether there have been quality failures reported by the World Health Organization (WHO) regional network. Prof. Jayaratne also explained that WORs were requested after the tenders had been awarded (a process in which the NMRA was not involved), either by the SPC Chairman or the Health Ministry on behalf of the MSD. “The WOR then issued by the NMRA is not ‘blanket’ registration but only for one particular consignment,” he stressed, adding it is not an unlimited entry gate-pass. It is to meet an urgent need. | |
The best way to say that you found the home of your dreams is by finding it on Hitad.lk. We have listings for apartments for sale or rent in Sri Lanka, no matter what locale you're looking for! Whether you live in Colombo, Galle, Kandy, Matara, Jaffna and more - we've got them all!

