News
Backyard mask-makers cheat public while regulators stand back
Substandard face masks are being falsely sold as having been quality-certified or imported, prompting complaints from unhappy purchasers and manufacturers of certified masks, with a gap in government regulation being exploited.
Consumers say the masks emit a stench, cause rashes, have unevenly fixed straps and contain dust.
Rasika Priyani, a resident of Galle, said a three-ply face mask she used for about two hours caused skin irritation and redness on her face. The itchiness lasted the whole day, she said.
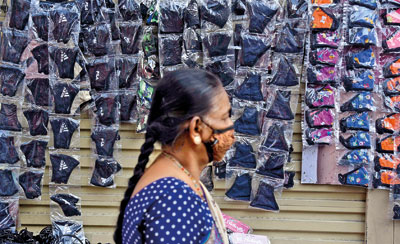
Little quality control: Masks with fake brand names for sale in Pettah. Pic by Akila Jayawardana
A three-wheeler driver who operates at Kiribathgoda, Sampath Madanayake, said he had to keep buying new masks as the straps of his masks grow loose.
“The masks available in the shops are useless. Sometimes I can’t breathe while using them. Other times, the straps break. I am a driver and I can’t stop everywhere to buy new masks,” he said.
He pointed out that as a transport provider he relies on masks as his primary safeguard from infection when dealing with multiple passengers every day.
These were only a few of many complaints made by consumers.
Mass-scale mask producers too raised concerns over the wellbeing of the consumers as well as the sales of their own products.
One such producer, Samantha Fernando, Chairman of private company, said the firm had lodged complaints at the Consumer Affairs Authority and its ministryand National Medicines Regulatory Authority (NMRA) about fake or substandard masks being distributed.
He said the complaint has also been forwarded to the Government Medical Officers Associations (GMOA) and the Director-General of Health Services, Dr. Asela Gunawardena.
Mr. Fernando said some brands are labelled as being made in China but are made locally.
He claimed these masks are being made in appalling conditions.

Masks being made in ad hoc manner
“Reputed local manufacturers are devising automated systems and using sterilisation methods to produce masks but others, who are making masks in backyard sheds, ignore all safety and hygienic measures and make substandard products. The authorities should stop these products reaching the market,” Mr. Fernando said.
He said that in his factory it is mandatory for workers to use masks at all times and to use protective gear and disinfect their environment.
“We make sure that our masks are uncontaminated and also obtain approval from NMRA and other international authorities,” he said.
The Sunday Times found that surgical mask cartons and boxes of K- 95 masks were falsely labelled “Made in China” while containing locally manufactured masks. Employees working in these establishments confirmed this.
The cartons had Chinese inscriptions that, when translated, made no sense. One three-ply mask carton in Chinese when translated read as: “The learning and students of the school and students, the opportunity to join together to seek more dance and dance to the end generation”.
Another box which had a Google translation of the Chinese wording reads as: “The communication is the use of Hua Ke want to agree with each other is to each Huahua on the Hewen to Communicate with heart and sow the Chinese”.
The NMRA acknowledged there were mask-makers who were not registered with the authority that were falsely printing “NMRA approved” on their products.
NMRA’s Regulatory Pharmacist Wasana Welipitiya said the authority can only regulate the quality and safety of equipment with a medical use. Thus, it can only regulate three-ply face “medical” masks and K-95 “medical grade” respirators used by medical personnel.
Ms Welipitiya said the authority does not have purview over non-medical three-ply face masks and K-95 respirators sold for civilian use. She said these products needed to be manufactured according to Sri Lanka Standards Institute (SLSI) standards and Consumer Affairs Authority (CAA) specifications.
Ms Welipitiya stressed the government needed to ensure non-medical face masks were indeed made according to SLSI standards and find a way to stop unhygienic masks entering the market.
An SLSI spokeswoman denied, however, that the institute had authority under its act of operation (SLSI Act No. 6, 1984) to check the quality of face masks.
“Masks are considered medical devices and the NMRA controls quality approval,” said the spokeswoman, who cannot be named due to separate proceedings.
She said the SLSI formulated two voluntary standards as Specifications for Single-use Medical Face Masks (SLS 1683: 2020) and Guidelines for Reusable Non-medical Face Masks (SLS 1675: 2020) but these were not mandatory. It was up to manufacturers to voluntarily adopt and implement these standards.
Face masks approved by the NMRA have obtained the authority’s Good Manufacturing Practice (GMP), Device Registration and Manufacturing licence. The authority follows World Health Organisation specifications to ensure quality.
“Some of the specifications are bacterial filtration efficiency, breathability, a design that doesn’t collapse against mouth, and fluid resistance,” Ms. Welipitiya said.
A source at the NMRA told The Sunday Times there were only three food and drug inspectors at the authority and the authority’s capacity to investigate was limited.
They said when a mask manufacturer sought registration, only an NMRA pharmacist visited the premises, not the food and drug inspectors, who enforce regulations. If the inspectors made the visits they could take action if hygiene conditions are not met.
Ms. Welipitiya rejected claims by consumers that the NMRA has not taken action on substandard medical masks, saying the authority has acted on complaints received.
NMRA Chief Food and Drug Inspector Amith Perera also said he was aware of substandard masks in the market.
“There was a detection of 200 substandard face masks sold as three-ply medical face masks in Kiribathgoda last year. Those were garment factory-made masks,” Mr. Fernando said. The masks, made of cloth-like material, were seized and destroyed and the garment factory owner had been issued a warning.
 A CAA spokesman said it had not received complaints about masks and was unable to make raids without a complaint being received.
A CAA spokesman said it had not received complaints about masks and was unable to make raids without a complaint being received.
The Sunday Times learned, however, that a complaint was lodged with the CAA on April 21 and the body’s Competition Promotion Division had stamped it.
Ms. Welipitiya asked consumers to limit the use of fabric face masks. She said that people should not reuse face masks repeatedly after washing them in a washing machine. There can be tears and gaps created when a face mask is washed that way, she said.
“The mask should allow breathing and also filter bacteria,” she said.
The Secretary of the Ministry of Technology, Jayantha de Silva, declined to comment on the sale of unhygienic masks.


