News
COVID-19: Where Sri Lanka stands in vaccine scenario
Hemas Pharmaceuticals, the local agent of both AstraZeneca and Pfizer, two major anti-COVID-19 vaccine manufacturers, has approached the National Medicines Regulatory Authority (NMRA), the Sunday Times understands.
“AstraZeneca and Pfizer are now working directly with the NMRA and are in the process of submitting their dossiers,” said the Group CEO of Hemas Holdings, Kasturi Chellaraja Wilson, explaining that all logistics including the supply chain will be handled by Hemas which is working with partners who are specialists in their respective fields.

Prof. Asita de Silva
The British-Swedish company AstraZeneca along with the University of Oxford has manufactured ‘ChAdOx1 nCoV-19 (AZD1222)’which is a two-dose vaccine, taken four weeks apart, as a muscle injection.The company says that clinical data show 90% efficacy.
The New York-based Pfizer and German company BioNTech have manufactured ‘BNT162b2’ which is a two-dose vaccine, taken three weeks apart, as a muscle injection. The company states that clinical data report 95% efficacy.
“We have asked for the clinical trial data dossiers,”confirmed NMRA Chairman Prof. Asita de Silva, adding that no dossier has been submitted yet.
While no vaccine-candidate world over has still been endorsed for emergency use by the World Health Organization (WHO) which is studying trial data, Sri Lanka has not granted any provisional registration to any vaccine against COVID-19.
The Pfizer vaccine has got emergency-user listing (EUL) in the United Kingdom (UK), the United States of America (USA), Canada and some European countries, it is learnt.

Prof. Neelika Malavige
Whatever procurement pathway Sri Lanka takes for vaccines against COVID-19 (either through COVAX or agents of manufacturing companies), the NMRA registration is mandatory, reiterates Prof. de Silva and that would also be dependent on the endorsement by the WHO.
Asked when the vaccines for 20% of Sri Lanka’s population through COVAX would come into the country, he said that the “first wave” of injections (of the 4.2 million injections due) may be brought in early February, next year according to the WHO.
The government has secured vaccines for 20% of the population through COVAX(COVID-19 Vaccine Global Access), a global initiative, which works with vaccine manufacturers to provide countries worldwide equitable access to safe and effective vaccines, once they are licensed and approved. COVAX is co-led by GAVI (Vaccine Alliance); the Coalition for Epidemic Preparedness Innovations and the WHO.
The Sunday Times this week takes an in-depth look at the vaccine scenario and where Sri Lanka stands.
There are two systems through which vaccines can usually be brought into Sri Lanka but under both these systems, the vaccines, which are endorsed by the WHO, also have to be mandatorily registered by the NMRA, after an independent evaluation.
The two systems are:
Once a vaccine is endorsed by the WHO and the regulatory authority of the country where the vaccine has been produced registers it, Sri Lanka would hold extensive discussions with input from all technical experts and decide whether it would be introduced to the country by the authorities.
Agents representing vaccine manufacturers can seek registration from the NMRA directly and once again it would carry out the relevant checks, a pre-requisite being endorsement by the WHO, and register it for use in the private sector.

Dr. Nihal Abeysinghe
NMRA’s Prof. de Silva said that in this unprecedented pandemic situation, the WHO is looking at trial data, while considering whether two stringent regulatory authorities in two separate geographical regions [such as the Food and Drug Authority (FDA) of the US and the European Medicines Agency (EMA) of the European Union (EU) or the Medicines and Healthcare products Regulatory Agency (MHRA) of the UK] have given approval for EUL. This is to support less mature regulatory authorities to decide which vaccines they should register.
While the WHO has many dossiers on the main vaccine-candidates, the NMRA has been told that it is expecting the Russian dossier in January.
The Pfizer vaccine has got EUL from the US FDA, Canada’s regulatory authority, the UK’s MHRA and the EU’s EMAand Moderna’s vaccine (mRNA-1273)by the US FDA. These regulatory authorities have been carrying out rolling reviews of the clinical trial data from the beginning, as this is a pandemic situation, says Prof. de Silva.
“The NMRA, meanwhile, has undergone weekly training sessions held by the WHO since September on regulatory oversight processes. We will follow these processes stringently with the support of the WHO when we give provisional registration to any vaccine against COVID-19,” he said.
He adds that last week (on December 15), the WHO released the ‘Safety Review Guidelines’ and the NMRA is “ready” for reviews of vaccine-candidates with expert panels in place.
Explaining the status of Sri Lanka with regard to the COVAX initiative, former Chief Epidemiologist Dr. Nihal Abeysinghe says that Sri Lanka is among the 92 low and middle-income countries, which come under the ‘Advance Market Commitment’ (AMC) facility of COVAX. As such, Sri Lanka has been assured of vaccines for 20% of its people and these doses would be free of charge.
So far, COVAX has secured 2 billion doses (of which it already has in hand over 1 billion doses) from different manufacturers.
As of December 18, COVAX had:
170 million doses from Oxford University/AstraZeneca
500m from Johnson & Johnson
200m from India’s Serum Institute, with an option of securing another 900m. The Serum Institute is working with AstraZeneca as well as Novavax
200m from Sanofi/GlaxoSmithKline (GSK)
Getting down to the nitty-gritty, Dr. Abeysinghe who is part of the Vaccine Committee says that each country coming under the AMC facility has to submit a two-prong application.
The application’s two-prongs are: ‘Vaccine request’ which had to be submitted by December 7 which Sri Lanka has done &the ‘indemnification agreement’ due to be submitted by Jan 7.
Dr.Abeysinghe says that under ‘Vaccine request’ for ‘Vaccine characteristics’, Sri Lanka has given the following in priority order:
With the intention of protecting the country’s strong Expanded Programme on Immunization (EPI) and also not wasting valuable funds, Sri Lanka has requested WHO-endorsed vaccines.
Vaccines which can be stored at 2-8°C (the country’s traditional cold chain) all along – from importation to the jab being given. This is because for any other vaccine, the country would have to re-haul its storage facilities at great cost. The country has –20°C facilities only in its bulk warehouse and not elsewhere and even here large stocks cannot be stored.
Low price.
Vaccines using the viral vector.
Vaccines approved by stringent regulators in manufacturing countries.
Vaccines where fewer doses have to be given – ideally a single dose but if not twodoses and no more than that.
Inactivated vaccine.
Fewer doses per vial.
Vaccines which are on emergency-use listing.
Under the ‘indemnification agreement’, which is like ‘insurance’, Dr. Abeysinghe adds that it deals with how the country would handle any adverse effect which may crop up by chance among the people who have been vaccinated
Depending on the ‘priority listing’ of vaccine characteristics, COVAX assigns a place among the 92 in the AMC facility and tries to match each country’s position.
“If we don’t accept what is offered after such a match, then we go down in the list when being issued with vaccines,” adds Dr. Abeysinghe.
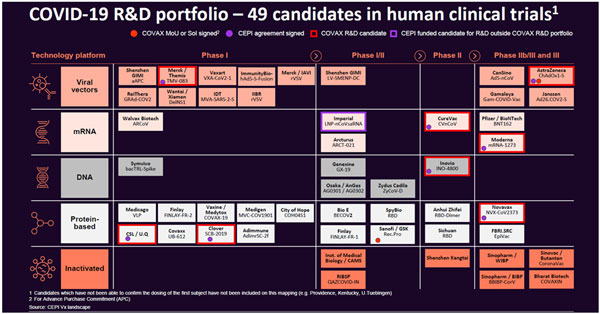
| WHO on vaccines and Sri Lanka Here are some queries that the Sunday Times posed to the WHO Representative to Sri Lanka, Dr. Razia Pendse. Q. Has the WHO prequalified a vaccine or is the WHO hoping to do so soon? If so, which ones? Currently, WHO has not prequalified a vaccine or approved emergency use. The draft landscape of COVID-19 vaccine candidates (please see ‘WHO’s COVID-19 R&D Portfolio on our website sundaytimes.lk) contains information on vaccine candidates collected through public information (e.g. clinical trial registries) and information that was directly provided by vaccine developers to WHO. The vaccines are listed in the table as per the clinical evaluation phase at the time point when the table is constructed (i.e. Preclinical, Phase 1, Phase 2& Phase 3). At a given stage, candidates are listed by alphabetical order; this does not reflect any preference or opinion on their potential to be successful (safe and effective). The landscape is generally updated twice a week. WHO has an emergency use and listing procedure in place to assess novel products to be used in epidemic conditions and speed up access for patients (WHO Emergency Use Listing of Vaccines, Q&A for Guidelines on Emergency Use Listing Procedure, Q&A for Use of Emergency Use Listing procedure for vaccines against Covid-19). Q. When would Sri Lanka get the promised vaccines for 20% of its population through COVAX? Which vaccines and how much would it cost? What of vaccines for the balance population? As of today, 190 countries and economies have signed up for COVAX, including Sri Lanka. All countries that have signed up for COVAX, are eligible to receive an initial proportional allocation of doses. Countries will progressively receive doses until all countries reach 20% of their population. We expect doses to begin arriving in countries in the middle of 2021. As these first doses will be limited, we will need to prioritize them for vulnerable groups like health workers and older people. To address this, WHO has developed a Fair Allocation Framework with partners and MemberStates. This will occur in the following way: Phase 1 – An initial proportional allocation of doses to countries. Countries will progressively receive doses until all countries reach 20% of their population. Phase 2 - to expand coverage to other populations. If severe supply constraints persist, a weighted allocation approach would be adopted, taking account of a country’s COVID threat and vulnerability. Which vaccine will depend on availability and the country’s submitted request to GAVI.
| |
| Two worrisome strains abroad: Experts & WHO/Europe explain details Concern is mounting about the new strain (scientifically known as clade) of the SARS-CoV-2 virus in the United Kingdom (UK), with flights from there to Sri Lanka being halted as of Wednesday (December 23).Reiterating that there are two strains of the virus which are worrisome, Prof. Neelika Malavige who heads the Department of Immunology & Molecular Medicine of the Sri Jayewardenepura University says that one strain is in the UK and the other is in South Africa.“They both seem equally bad,” she says, going into detail how any micro-organism such as a virus keeps evolving to replicate itself and be transmissible in a better way. Initially, SARS-CoV-2 was identified in Wuhan, which then spread across the world mutating (evolving) all the while. As it evolves, it becomes fitter and better at spreading the disease. Spotlighting the latest strain in the UK, Prof. Malavige says that the mutation has occurred in the spike-protein (S) which attaches itself to the host. Preliminary investigations by UK scientists have found that this strain is more transmissible (able to spread from person-to-person more). The earlier strains were bad enough and this could be worse. She says that while the UK’s new strain emerged in late September, the one in South Africa is “quite recent”. Pointing out that whether a new strain is circulating in Sri Lanka can only be ascertained by sequencing, she says that her laboratory has been conducting sequencing on strains in Sri Lanka since March. “We have not seen the UK strain here up to November. We hope to do more sequencing soon.” Prof. Malavige add: “The new UK strain has several mutations but those mutations are unlikely to affect the detection of the virus by RT-PCR kits used in Sri Lanka as they target mostly the N, ORF1 and sometimes the E gene. If it will affect the detection by antigen tests should be investigated. “There is an RT-PCR kit called Taqpath, which is widely used in many countries for SARS-CoV-2 detection, including our lab. This PCR kit uses, 3 genes targeting, S, ORF1 and N and the UK new variant, escapes detection by the S gene and gives a positive result for the other two genes. Therefore, if this result occurs with this RT-PCR kit, one should be mindful that we might be detecting the new UK strain. However, confirmation of viral sequences can only be carried out by whole genomic sequencing, which we continue to do on our viral strains.” When asked whether the new strain could have an impact on the efficacy of vaccines, Prof.Malavige added that she does not think so. The vaccines are likely to work because a few changes in the protein are unlikely to affect efficacy. “However, it would be important to carry out experiments to be sure that it has not affected efficacy, which I believe will be done soon,” she added. Former Chief Epidemiologist Dr. Nihal Abeysinghe said that mutations (adaptations) of the SARS-CoV-2 virus should be expected. These adaptations could work either way – the infectivity of the mutated virus strains could be more or less. In response to a query from the Sunday Times, the Europe Press Office of the World Health Organization (WHO) states that all viruses, including the COVID-19 virus, change over time. It states: “We are still learning about the significance of these or other mutations on public health. Initial epidemiologic analyses and modelling conducted by the UK indicate that there may be a change in the transmissibility of this new SARS-CoV-2 variant, as well as reduced performance in diagnostic tests that use the S gene target. “There is so far no evidence of changes in severity, antibody response or vaccine efficacy. The UK is undertaking further investigations to confirm initial findings and to extend our understanding of this new strain. It is important to emphasize that continued circulation is likely to result in many more mutations over time. This is why it is key that any new variants are identified through sequencing and assessed for their public health impact. “Countries need to continue increasing sequencing of SARS-CoV-2 viral isolates, upload to GISAID (a global science initiative providing open-access to genomic data of influenza and COVID-19 viruses) and report any new mutations to WHO through their International Health Regulations National Focal Points.”
|

Kasturi Chellaraja Wilson
‘No shortcuts in vaccine manufacture’
With vaccines on the mind of one and all in Sri Lanka, it is Jayewardenepura University’s Prof. Neelika Malavige who is heading the Department of Immunology &Molecular Medicine, who answers some crucial questions for the Sunday Times.
Have there been any ‘shortcuts’ when manufacturing the vaccines against COVID-19?
Prof.Malavige says that this is a concern expressed by many, as usually the manufacture of vaccines takes about 5-10 years. But, she assures, no shortcuts have been taken in the vaccines against COVID-19 even though they have come out in less than one year.
She takes the Sunday Times through the step-by-step processes that companies follow after securing ethics approval:
Pre-clinical stage – this involves animal studies and the vaccine-candidates have gone through this.
Phase 1 clinical trials – this involves testing on fewer than 100 completely healthy adults and the vaccine-candidates have gone through this. The data on these trials have been submitted.
Phase 2 clinical trials – these trials to test ‘safety’ and ‘immunogenicity’ are carried out on hundreds of people of the ‘intended’ group. A factor to remember is that in the case of vaccines against COVID-19, they are not intended for children as of now.
Phase 3 clinical trials – these trials are carried out in thousands (around 40,000 to 50,000) in multi-centres to test both ‘safety’ and ‘efficacy’. Here the vaccine-candidate would be given to adults with co-morbidities such as diabetes and adults of all ages. However, pregnant and lactating mothers are not included as they are considered a special group.
After all these stages, the trial data are closely evaluated, reiterates Prof. Malavige and it is only after the Phase 3 data are evaluated and approvals are granted by regulatory authorities that the manufacturing begins of the approved vaccine usually.
Explaining that in the case of the vaccines against COVID-19, there was an overlap among Phases 1, 2 & 3 because the pandemic is an emergency of proportions not experienced before. While in Phase 3 trials, the manufacturers also took a “huge chance” and began manufacturing during data evaluations. In case, the data were rejected they would have suffered a big loss as all those vaccines would have had to be discarded.
Next Prof. Malavige goes onto the last phase which is an ongoing one:
Phase 4 – Post-marketing surveillance which happens continuously to check out the long-term safety data.
However, there being an emergency situation with widespread disease and death, the companies have had no option but to side-step this phase, initially, she adds.
The Sunday Times understands that only Pfizer, Moderna and Oxford-AstraZeneca have presented their Phase 3 findings in peer-reviewed journals.
With regard to Sri Lanka, Prof. Malavigeechoed the views of other experts that “we would await WHO endorsement”.
The US and UK started jabbing people with the Pfizer vaccine in early December, while India and Bangladesh are hoping to begin administering the vaccine in January.