News
In the best interest of patients, we give all sides of the story
View(s):The Sunday Times replies;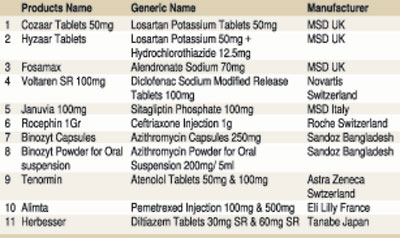
Requests by the Sunday Times for one-on-one interviews with the Health Minister and the Chairperson of the National Medicines Regulatory Authority (NMRA) failed to elicit a response from either.
In the rebuttal by the NMRA team, however, it is claimed that the article is based on information purportedly obtained from “unnamed sources”.
The Sunday Times would like to point out that the Sri Lanka Chamber of Pharmaceutical Industry (SLCPI), the very next day (October 15) confirmed the withdrawal of the drugs mentioned in the article. (See below for release from SLCPI)
The label of ‘essential’ for the 48 drugs of which the prices were slashed was not given by the newspaper but in general by the authorities.
We would also like to point out that many of the bullet points mentioned by the NMRA with regard to the loading of the Cost Insurance Freight (CIF) prices; the process through which the prices were reduced; the composition of the Pricing Committee; the analysis of market data from the International Marketing Services (IMS); and the World Health Organisation’s praise for the NMRA’s revised pricing policy have been reported in detail by the Sunday Times.
The Sunday Times would like to put on record the following instances when the positive aspects of the price-cuts of drugs were highlighted;
- An article headlined ‘Vital drug prices to come down at last’ (September 25, 2016) quoting both Health Minister Dr. Rajitha Senaratne and NMRA Chairperson Prof. Asita de Silva.
- ‘Drug price slash: A quick fix eyewash’ (October 30, 2016)
- ‘Price control on 48 drugs: Hotlines kept busy’ (November 6, 2016)
- ‘More drug prices slashed: Good move or rushed move?’ (August 5, 2018)
- ‘Price-cuts for 25 medicines soon’ (August 19, 2018)
It was in the article on August 5 that Dr. Ananda Wijewickrama rejected views that branded drug companies would pull out. He did not indicate to the Sunday Times that in fact by late 2016/early 2017, some brands had pulled out — for whatever reason.
The very same article also gave wide coverage to the Generics vs Brands debate and his argument that big failures among generics was a “boru kathawak” (false talk), as generics are used in the state sector. However, when asked whether there has been an audit of quality failures of drugs in the state sector, he said he did not know.
Meanwhile, in the Sunday Times article on August 19, which was based on an exclusive two-hour interview with Prof. Asita de Silva (at which were present NMRA CEO Dr. Kamal Jayasinghe and NMRA Member Dr. LakKumar Fernando), the newspaper was told that the prices of 15 more essential medicines including neurological and diabetes drugs and life-saving intravenous antibiotics as well as 10 anti-cancer drugs were to be slashed shortly.
This article spread over nearly a full-page in the Sunday Times also highlighted different aspects including ‘Overall use and access to quality medicines rise, while market share of innovator brands also increase’; ‘How the 10 anti-cancer medicines were chosen’; and ‘Praise from the WHO’.
While expressing views that there can be profit for the pharmaceutical industry but there should be no profiteering, which the Sunday Times highlighted in its headline as well, it was also in this article that this newspaper quoted that “if you look at the analysis, we (NMRA) feel our task of providing increased access to quality assured medicines has happened……..no pharmaceutical company has pulled out although there was a major clamour that they would do so. There was one case of a lipid-lowering brand being withdrawn but it is back in the market now. These are the scare-mongering tactics of some interested parties”.
The Sunday Times in this August 2018 interview was not told about the drugs withdrawn in late 2016 (as substantiated by the SLCPI subsequently through a media release this week), for whatever reason — be it market share, price etc.
We would also like to point out that the rebuttal is silent on ‘Rocephin’ 1Gr (ceftriaxone Injection 1g) for meningitis and ‘Alimta’ (Pemetrexed Injection 100mg and 500mg), an oncology medication for lung cancer.
Without a doubt, some of the price-cuts of drugs have helped the patients, but the Sunday Times, in the interest of all patients, has been giving the other or all sides of the story to make the drug price reductions more workable and sustainable. This is to ensure safe and effective drugs at reasonable prices for the public.
Sri Lanka Chamber of Pharmaceutical Industry
- Pharmaceutical Industry withdraws 11 drugs from market
- Importing them has become commercially unviable
- Notes with concern that biggest setback is for patients
October 15, 2018: The Sri Lanka Chamber of Pharmaceutical Industry (SLCPI) in a statement to media said that despite persistent appeals, the Government has failed to address the issue of a fair and proper pricing mechanism for pharmaceutical products in light of the sharply depreciating Sri Lankan Rupee against the US Dollar. With over 85% of pharma products being imported, both the Ministry of Health and the National Medicines Regulatory Authority (NMRA) have remained willful in depriving the industry of adjusting pricing of products as per the prevailing exchange rate, SLCPI noted with concern.
As such, the pharmaceutical products shown in the table will no longer be available in the market as it is not commercially viable to import them.
Some of the above drugs were withdrawn in 2016 due to the price ceiling and in the absence of a pricing formula.
SLCPI notes with distress that discontinuation of pharmaceuticals implicates multiple parties such as the manufacturing firms, patients, providers, pharmacies and the health authorities. The biggest setback would be for the patients who are adversely affected if the drug product that provided excellent therapeutic benefits and resulted in positive health outcomes is no longer available in the market.

