News
Rolled up sleeves as rollout of vaccines for health workers begins
The 500,000 doses of COVISHIELD were handed over to President Gotabaya Rajapaksa by Indian High Commissioner Gopal Baglay soon after an Air India flight brought the stocks from the Serum Institute on Duruthu Poya under India’s ‘VaccineMaithri’ initiative.
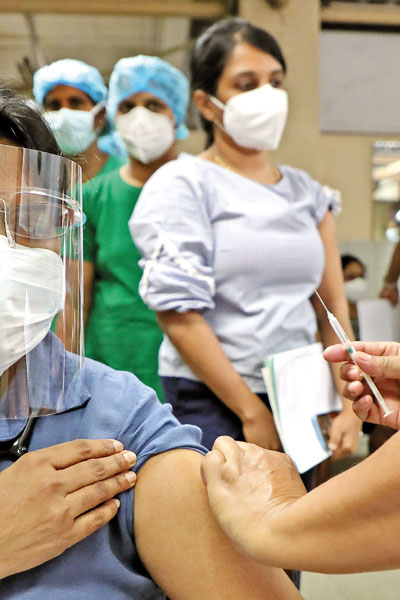
Waiting in line for the jab: Scene at the NHSL on Friday Pix by M A Pushpa Kumara
The rollout and administration began on Friday symbolically at the National Institute of Infectious Diseases (NIID) with senior Consultant Physician Dr. Ananda Wijewickrama who is treating COVID-19 patients getting the first jab. The second dose will follow four weeks later.
The Sunday Times saw firsthand as staff at the National Hospital of Sri Lanka (NHSL) stood in line for their vaccination in the ground floor of the Accident Service, while on the other side not only masked and with visors but also in full protective gear, other staff attended to patients. The largest number of people, 1,886 in all, was vaccinated at the NHSL on Friday.
“We are hoping to vaccinate all 10,000 of our staff including cleaners etc,” said the Deputy Director General of the 3,431-bed NHSL, Dr. Kumara Wickramasinghe, adding that he is due to get the jab today (Sunday).
As some staff pulled up their sleeves and others slipped their arms out of their sleeves, got the jab and were directed to sit awhile (about 20 minutes) before moving away from the vaccination area, Dr. Wickramasinghe explained that the process included registration, seeking consent, identification of any contraindications, vaccination and finally observation for 20 minutes. If there is any allergic reaction, there is staff to attend to it immediately, with emergency trolleys and beds close-by.
The NHSL target is to give the first dose to all within three to four days.
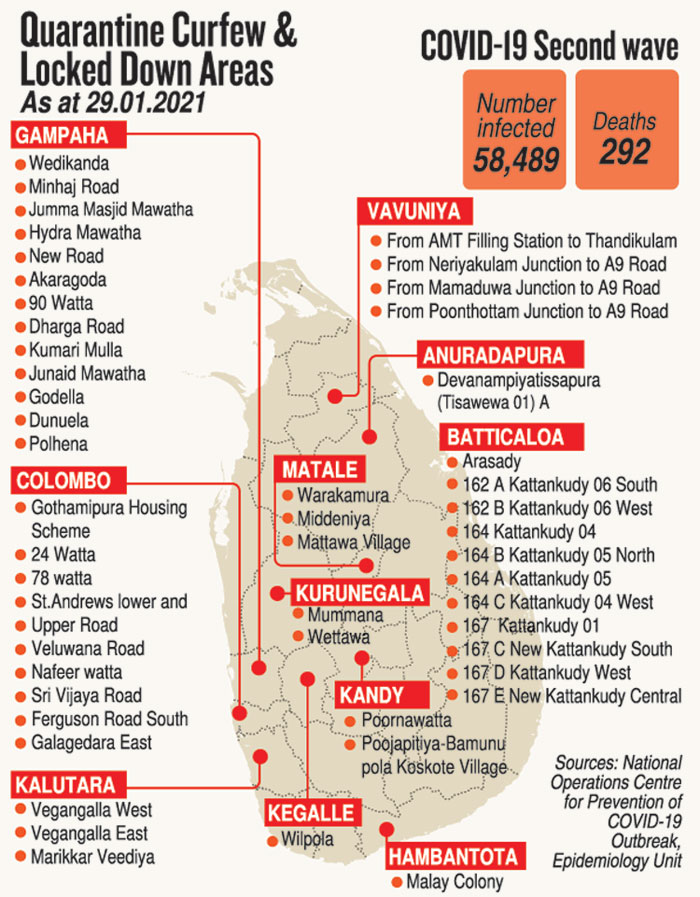
This was as the number of COVID-19 positive people in the second wave in the country reached 58,489 and deaths 292, with Friday’s positive number being 859. Those in hospitals totalled 6,750.
The total number of deaths from COVID-19 reached 305 in Sri Lanka, as the death toll around the world this week hit 2,182,867 and the toll in America alone surpassed 1 million.
Vaccine
Known in the United Kingdom as ChAdOx1 nCoV-19 (vaccine AZD1222) and developed by the Oxford University-AstraZeneca pharmaceutical company, the same vaccine is being manufactured as COVISHIELD by the Serum Institute at its factory in Pune. It is a two-dose vaccine given four weeks apart as an intramuscular injection.
India has given these 500,000 doses free of charge to Sri Lanka under its vaccine diplomacy efforts to neighbouring countries including the Maldives (100,000 doses for this country which has a population of 530,953), Bangladesh (7 million doses for a country which has 164 million people) and Bhutan (150,000 doses for a country which has a population of 776,434).
“The vaccinations started on Friday with stocks being distributed to healthcare institutions not only in Colombo but all districts as deliveries went along seven different routes,” the Deputy Director General of Health Services (Public Health Services II), Dr. Susie Perera told the Sunday Times.
She pointed out that healthcare staff and others involved in the maintenance of a hospital such as cleaning, security and canteen people are on the vaccination list. It is being given in batches (“katti kattiwalata”) according to lists drawn up by the heads of these institutions. All those in all major hospitals, Divisional Hospitals A and B and Medical Officers of Health (MOH) offices are due to get the jabs.

Dr. Kumara Wickramasinghe
At the end of Friday, Chief Epidemiologist Dr. Sudath Samaraweera announced that 5,286 personnel in 10 hospitals and other institutions were vaccinated and no side-effects had been reported.
Friday’s vaccinations were at the NHSL – 1,886; the Colombo North (Ragama) Teaching Hospital – 803; the Colombo South (Kalubowila) Teaching Hospital – 781; the Army Hospital – 600; Panagoda Army Camp – 400; the Lady Ridgeway Hospital for Children, Colombo – 382; the Homagama Base Hospital – 190; the Colombo East (Mulleriyawa) Base Hospital – 108; the NIID [better known as the Infectious Diseases Hospital (IDH)], Angoda – 80; and the Welisara Navy Camp – 56.
“We have a real-time electronic Information System,” says Dr. Perera so that this “huge” operation is carried out systematically. Many people are handling the different aspects of this programme while she is heading the logistics team.
She said that COVAX (COVID-19 Vaccine Global Access) is due to give Sri Lanka vaccines for 20% of the eligible population and then there is also the facility of getting more vaccines through COVAX on a cost-sharing basis.
COVAX is a global initiative which works with vaccine manufacturers to provide countries worldwide equitable access to safe and effective vaccines once they are approved and licensed. It is co-led by GAVI (the Vaccine Alliance), the Coalition for Epidemic Preparedness Innovation (CEPI) and the World Health Organization (WHO).
Meanwhile, the Head of the Presidential Task Force on Vaccines, Lalith Weeratunga told a briefing on Wednesday that the government and Health Ministry take full responsibility with regard to administering this vaccine as the benefits outweigh any risks there may be and COVISHIELD is the first step towards vaccinating the people.
He said that frontline health workers, followed by selected officers from the police and tri-forces as they support COVID-19 management operations are on the priority list. They amount to around 150,000 frontline health staff and about 120,000 personnel from the police and tri-forces.
The priority list also includes those over 60 years of age and those with co-morbidities but the Sunday Times learns that they will not come into this round of vaccination as the total who can be vaccinated with this stock is 250,000.
While electoral lists are being used to identify those on the priority list, it is learnt that the authorities are also hoping to run a campaign to catch anyone who may have fallen through the net.
Essential process of vaccine registration
Referring to the essential process of registration for the protection of people against substandard medicines or vaccines, Mr. Weeratunga said: “We did not influence the National Medicines Regulatory Authority (NMRA) to speed up the registration process. The NMRA has granted permission to use it as an emergency vaccine. We have lined ourselves up on the world’s competitive stage to get the vaccines from various institutes. Right now, we only have approval for the AstraZeneca vaccine for which we have placed orders.”
He explained that if Russia’s Sputnik-V and China’s Sinopharm vaccines get approved and that would be based on data generated from clinical trials, then Sri Lanka would have a combination of vaccines.
“Tentatively, I can say that at least 50-60% of the target group we are looking at can be vaccinated within the course of the year. It should be a safe time-table. China has said that they would donate 300,000 doses of the Sinopharm vaccine. Once the NMRA goes through its studies and approves the vaccine we can start the process,” he said.
The Sunday Times learns that the NMRA permission was granted to COVISHIELD on the basis of the emergency use listing given by the stringent drug regulatory authority of the United Kingdom (the Medicines and Healthcare products Regulatory Agency – MHRA), the preclinical and clinical trial data published in peer-reviewed journals and evaluations of the dossier by two teams of the NMRA which looked at clinical efficacy and safety data.
The NMRA has also submitted its findings and the pathway taken in granting such permission to the National Immunization Technical Advisory Group (NITAG) which is part of the Non-Communicable Diseases (NCDs) Committee of the Health Ministry.
Those travelling abroad
With regard to vaccinating people travelling out of the country, Task Force Head Lalith Weeratunga said that it is a different concept from the planned activity. “We first have to go through certain layers of our population and they have to be carefully mapped out. Right now we do not have the facility but it might happen later when we have an adequate amount of vaccines and storage. It could then happen as a parallel activity.”
Consultant Epidemiologist Dr. Samitha Ginige said that health personnel have got adequate training in administering the vaccine. In terms of human resources, there is no issue for the vaccine rollout.
He stressed that the success of a vaccination programme can be measured by the maintenance of vaccine quality. Vaccines are very sensitive to temperature changes. From the day a vaccine has been manufactured and till the moment it is administered, the required temperature should be maintained. This is the challenge but for Sri Lanka it will not be, as COVISHIELD has to be stored between 2-8°C and in Sri Lanka all vaccines are stored at this temperature.
“By Thursday evening, we prepared the vaccines and the necessary tool kits to be sent to the other districts, keeping the doses to the required temperature,” he added.
The Sunday Times understands that adequate syringes for these injections have to be made ready as the vaccine doses come as stand-alone products.
Private sector involvement
Task Force Head Lalith Weeratunga said that the National Vaccination Programme does not factor in the private hospitals to administer the vaccine, particularly not this one as it is an emergency situation. At the optimum level of operations, the Health Ministry envisions having about 4,000 vaccination points including the 1,050 hospitals.
“Post-vaccination after-care is needed as some people may experience fainting, dizziness and nausea. When you factor all these in, it is not advisable for the private sector to be involved. The private sector has said that the cost for the vaccination of their employees will be borne by them. However, the government is administering the vaccine free of charge for at least six to nine months. First we are trying to focus on the critical layers of the population and after about two months, we hope to look into the alternate paths,” he added.
(Please see ‘Frequently asked questions on COVID-19 vaccination’ by the WHO )
| First supply of vaccines under COVAX next month As Sri Lanka embraced the vaccination programme against COVID-19 on Friday, the Sunday Times understands that the first supply of vaccines under the COVAX programme may be received in the country in the 2nd or 3rd week of February (next month). Sources however said that they did not know which type of vaccine the country would get but assured that it would be those which have been approved by stringent regulators elsewhere in the world. In another development, it is learnt that the British pharmaceutical company, AstraZeneca which developed ChAdOx1 nCoV-19 (vaccine AZD1222) with Oxford University, has given the green light to India’s Serum Institute to launch commercial production of the same vaccine under the name COVISHIELD. Sri Lanka has sent an expression of interest to buy about 2-3 million doses of COVISHIELD from the Serum Institute, it is understood.
| |
| 4 essential pillars that are interlinkedThere are four essential pillars on which the prevention and control of COVID-19 are based, reiterated by the Deputy Director General of Public Health Services II, Dr. Susie Perera. All need to be strong, otherwise it will not work, she said. They are:
“All these four pillars are strongly interlinked. If one collapses the whole thing will collapse. Then it would be like frantically trying to mop up the mess after leaving the tap open full blast,” adds Dr. Perera. |
MRI in forefront of battle against COVID as it marks 121st anniversary today
Celebrating the first anniversary when they put the shoulder to the wheel to help in the battle against COVID-19, the Medical Research Institute (MRI) looks forward to contributing more towards the national good.
Consultant Virologist of the MRI Dr. Jude Jayamaha says that the very first case of COVID-19 in the country (the Chinese woman tourist) was detected by in-house RT-PCR testing at the MRI. She was detected as being positive on January 27, last year (2020), after establishing the test for SARS-CoV-2 on January 26. In the region, Sri Lanka was the second to begin such testing.
Since then, the MRI has worked 365 days, totting up nearly 193,000 ‘gold standard’ RT-PCR (Reverse-Transcription Polymerase Chain Reaction) tests up to now, performing around 1,500 tests daily, he says.
Along with the MRI, currently 31 laboratories across the country – state, private and universities – are conducting these tests. Just this Wednesday, this collective effort resulted in 19,620 RT-PCR tests.
The MRI, meanwhile, is in the process of automating antibody testing which it is now doing manually. This is ELISA (enzyme-linked immunosorbent assay), a serology assay to check people for antibodies which would indicate whether they have already been exposed to the virus.
The MRI has also assisted SLINTEC (Sri Lanka Institute of Nanotechnology) to validate the rapid PCR LAMP (loop-mediated isothermal amplification) test kit which is now awaiting regulatory approval.
It is also conducting important research on the effects of black tea and immune responses of COVID-19 positive patients.
As Dr. Jayamaha pays tribute to all those, internally and externally, who have supported MRI to reach these goals, he adds that the MRI which was born as the De Soysa Bacteriology Institute way back in 1900 is celebrating its 121st anniversary today (January 31).
On vaccinating expectant and breastfeeding mothers

Dr. Sanath Lanerolle
As vaccination against COVID-19 began this week, a senior obstetrician looked at the need to vaccinate expectant mothers as well as women who are breastfeeding babies.
The President of the Perinatal Society and President-elect of the Sri Lanka College of Obstetricians & Gynaecologists, Dr. Sanath Lanerolle told the Sunday Times that pregnant women and women who are breastfeeding are already routinely and safely offered other vaccines in pregnancy.
Some of the examples he cites are vaccines that protect them against influenza and whooping cough, as he states that many of these vaccines also protect their babies from infection.
Pointing out that pregnant women have however not been part of specific clinical trials of COVID-19 vaccines, he says that different vaccines work in different ways and for some of the COVID-19 vaccines, previous studies on similar vaccines (such as the whooping cough or influenza vaccines) may provide some insight into effects in pregnancy and reassurance about safety.
Quoting the ‘updated advice’ issued by the Joint Committee on Vaccination and Immunization (JCVI) of the United Kingdom on December 30, last year, Dr. Lanerolle says that it confirmed that although the available data do not indicate any safety concern or harm to pregnancy, there is insufficient evidence to recommend the routine use of COVID-19 vaccines during pregnancy.
However, the JCVI is now taking a risk based approach that pregnant women with high-risk medical conditions, who meet the definition of being “clinically extremely vulnerable” should consider having the COVID-19 vaccine in pregnancy. This is because their underlying condition may put them at high risk of experiencing serious complications of COVID-19, it is understood.
“The benefits and risks of COVID-19 vaccination in pregnancy should be discussed on an individual basis. The discussion should include acknowledgement that, while there is no known risk associated with giving other non-live vaccines to pregnant women, there is no specific data as yet about the safety of COVID-19 vaccination in pregnancy,” he says.
According to Dr. Lanerolle those who fall into the clinically extremely vulnerable include pregnant women with significant heart disease (congenital or acquired), those with severe respiratory conditions including severe asthma etc.
Danger of variants entering country
The number of people affected by COVID-19 is creeping up in Sri Lanka and stringent measures are needed to prevent the new variants of the virus getting into the country as that would bring about disastrous consequences, warned health experts.
The opening up of the country to tourists is a big risk as the new variants in the United Kingdom (UK), South Africa and Brazil are found to be much more transmissible than the virus strains before them. The variant found in the UK is 50-70% more transmissible, it is learnt.
A source who follows world trends closely pointed out that the new variant found in South Africa is doing a “marvellous job” fighting off the neutralising antibodies that are usually produced when a person gets infected. This also raises the issue whether the variant would be hindrance to the protection that the vaccines against COVID-19 would provide.
The Sunday Times learns that in the face of the presence of a foreign substance (antigen) including disease-causing organisms such as viruses, a person’s immune system produces a protective protein – antibodies (immunoglobulins). The antibodies “latch” onto the virus to get rid of them.
“Early data suggest that the new variant found in South Africa (501Y.V2) is evading the virus killing-effect of antibodies found in the blood samples taken from people who have recovered from the previous wave of COVID-19 infections,” the source said, explaining that this may mean that the new variant is escaping the immunity a person developed against the first virus infection. Work is ongoing to establish whether this virus also escapes vaccine-induced immunity.
The variant detected in Brazil shares a key mutation with 501Y.V2 causing concern among scientists whether a similar situation is developing in that country, it is learnt.
The source said that the worry is that many countries are not carrying out systematic sequencing of viruses to detect the emergence of such variants, the UK and South Africa being exceptions that do. So it is possible that variant viruses of concern may be found in many other countries as well but are circulating undetected. In response to this threat, the World Health Organization’s International Health Regulations Emergency Committee has recently made a strong recommendation that global surveillance for virus variants by genetically sequencing more virus samples should be enhanced.
According to the British Medical Journal, the Brazilian variant (P.1 or known as VOC202101/02 in the UK), was first detected in travellers from Brazil who arrived in Japan in January. It involves 17 unique amino acid changes, three deletions, four synonymous mutations and one 4nt insertion. It has several mutations that are known to be biologically important, including E484K and N501Y and shares these two mutations with the viruses in South Africa.
The BMJ stated: “The N501Y mutation, which is also a feature of the English variant, has been linked to increased infectivity and virulence in mouse models. Meanwhile, the E484K mutation is thought to be associated with escape from the neutralising antibodies produced by the body against SARS-CoV-2. This mutation is present in the South African variant as well.
“The South African variant emerged around the same time as the English one, and has since been detected in at least 20 countries. Genomic data from South Africa suggested that the variant, known as 501Y.V2, quickly displaced other circulating lineages in the country as it appears to have a higher viral load and is therefore more transmissible. This variant shares similarities with the English and Brazilian variants in that it contains both the N501Y and E484K spike protein mutations.”