News
Diagnostic testing for COVID-19: A guide to basic principles
 Today the world faces a situation like none other than is known in living memory. Heart-rending images abound on TV and social media, as the new coronavirus SARS-CoV-2 burns its way across the world. Whilst we watch in shock and horror, scientists from far flung corners of the earth are racing to find ways to identify individuals who have been infected with SARS-CoV-2, as well as those who have recovered from COVID-19 disease.
Today the world faces a situation like none other than is known in living memory. Heart-rending images abound on TV and social media, as the new coronavirus SARS-CoV-2 burns its way across the world. Whilst we watch in shock and horror, scientists from far flung corners of the earth are racing to find ways to identify individuals who have been infected with SARS-CoV-2, as well as those who have recovered from COVID-19 disease.
Diagnostic testing is paramount if we are to resume a sense of normalcy in our lives, until a vaccine or cure is found. People who test positive for the virus can quarantine themselves, thus preventing the virus from spreading. In the case of COVID-19, some infected persons have no symptoms (asymptomatic), and can therefore spread the virus without even knowing they have it. Furthermore, the hope is (though not proven yet) that people who have recovered from COVID-19 will be immune to the deadly virus and thus help re-start the economy. To this end, two main types of molecular tests are available to diagnose and manage COVID-19. These techniques are well-known to medical scientists and doctors familiar with nucleic acid amplification tests (such as PCR) and antibody assays, but unknown to the public. This article describes the basic principles of these diagnostic tests that are being used globally to diagnose COVID-19.
The PCR Test
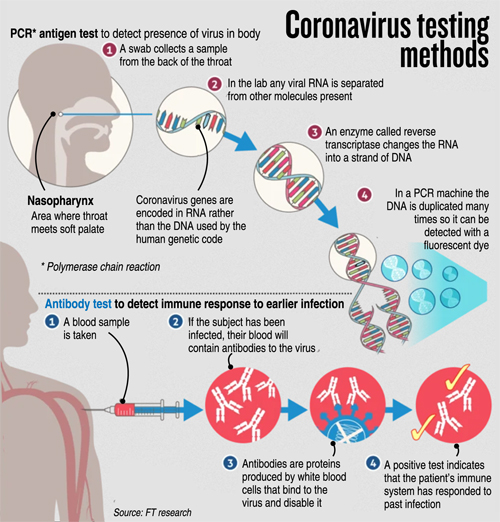
What is PCR? PCR (Polymerase Chain Reaction) is a well-established scientific technique that has been widely used for about 25 years in molecular diagnostics field.
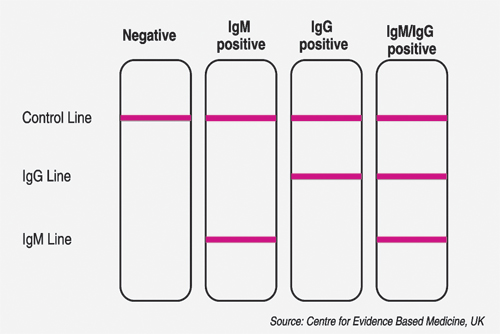
Lateral flow immunoassay test looks for IgM and IgG antibodies which are produced by the body in response to a virus invasion. These are very simple to read – a control line must appear to show the assay has worked correctly. If the antibodies are present in the patient blood sample, then the IgG and/or IgM lines will appear, indicating a positive test result.
PCR is fundamentally a nucleic acid (DNA) amplification method. To detect the novel SARS-CoV-2 virus, a special version of PCR is used, namely real-time RT-PCR. This type of test has frequently been used as a frontline test for COVID-19 as it directly tests for the presence of the virus genetic material, RNA. RT-PCR tests are sensitive and accurate and produce results in 6-8 hours on average. The technology is widely available, and many diagnostic and companies produce RT-PCR products, test kits and machines. Some RT-PCR tests are developed as an `all in one’ kit, reducing laboratory handling and potential for contamination. For SARS-CoV-2 RT-PCR testing, the FDA recommends test kits produced by certain companies only (for example Integrated DNA Technologies and Roche).
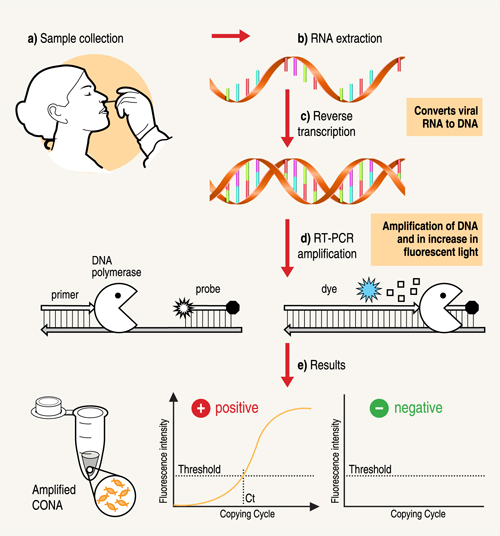
A nose or throat sample is taken using a swab – if the sample is collected from where the virus is shedding or multiplying, the accuracy of the test is improved. Chemicals are used to purify the viral RNA from other contaminants in the sample. Then, an enzyme (a molecule that speeds up chemical reactions), called Reverse Transcriptase, is used to copy the viral RNA to DNA. The DNA is then amplified using another enzyme DNA polymerase, which required short DNA molecules called primers to copy the viral DNA. The reaction is done in a PCR machine, which cycles the temperature (repeated heating and cooling cycles).
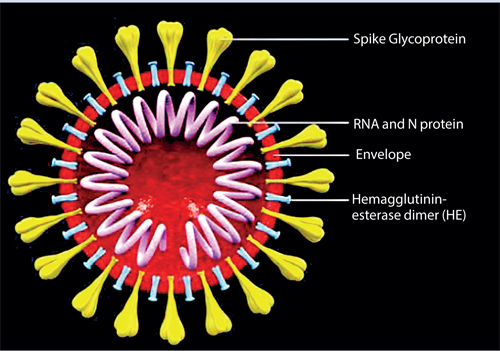
A virus contains the genetic material (either DNA or RNA) contained within an envelope made of fat and protein molecules. Certain viruses such as the coronavirus (SARS-Cov2) only contain RNA. This means that they rely on a hosts’ healthy cells to multiply and survive. Once inside the host cell, the virus uses its own genetic material to take control of and ‘re-programme’ the hosts cells to make them become virus-making factories
This results in billions of copies of viral DNA being made for each viral RNA strand that was originally present in the sample. In real-time PCR method, fluorescent probes (molecules which give out light when they absorb energy) are added to the mixture, which bind very specifically to a part of the viral genetic material. These probes emit fluorescent light at each PCR cycle. The fluorescence signal increases as more copies of DNA is produced. This fluorescence light can be read (quantified) by the PCR machine to produce the test result.
If the light produced reaches a certain threshold fluorescence (which is set above background levels), it is a positive test. If the virus were not present in the sample, the PCR test would not have made copies, so the fluorescence threshold is not reached – the test is then negative. The number of PCR temperature cycles that are required to reach fluorescence threshold is recorded and gives an estimate of how much virus was present originally in the patient sample.
Steps in the RT-PCR test: a) Specimen is taken from the nose or throat of patient b) RNA is extracted and c) is converted into DNA d) An enzyme, DNA polymerase, amplifies the DNA. The fluorescence increases as more and more copies of the virus DNA are made. (e) If the fluorescence level crosses the threshold, the test is positive.
Quality control procedures are extremely important to monitor the test performance and are crucial for the interpreting test results, as well as ensuring that the test kit components and all reagents (chemical substances) are working properly. The inclusion of both positive and negative controls with every batch of patient samples is a must. For example, for a negative control that has no genetic material in it should provide no amplification, whereas the positive control (a sample which is known to be positive for SARS-CoV-2 genetic material) should be amplified in the expected manner for a test to be valid.
What do the test results mean?
A positive PCR result means that the person the sample was taken from is currently infected by the virus. A negative PCR result could mean that the person is not currently infected by this virus, the virus is not present at the site the sample was taken from, the sample taken was of poor quality, or that it is too early, or too late in the infection to detect replicating virus. The RT-PCR test cannot detect if a person has had the virus and then cleared it after the disease ended, i.e. whether a person had the disease, as it only detects when active virus is present. Therefore, for a negative test result, if the patient has clinical symptoms of COVID-19, and/or contact with a confirmed positive case, re-testing a few days later is a must. These tests could also lead to false positives, if for example, specimens are contaminated, or the protocol is not followed appropriately.
What are the advantages of this test?
RT-PCR is accepted by medical researchers as a robust and well documented technique. It is highly sensitive and reliable if performed on a sample from an infected part of the body whilst an active infection is occurring. The disadvantages are that RT-PCR relies on detecting the virus itself and so it is possible to miss patients who have cleared virus and recovered from disease. RT-PCR for COVID-19 can only tell if a person is currently infected with SARS-CoV-2. A serious bottleneck in using this test for high-throughput COVID-19 testing is the major demand of testing kits and ancillary ingredients needed for sample preparation and testing.
 Antibody Testing
Antibody Testing
When a foreign invader, such a SARS-CoV-2, infiltrates the body, the immune system attacks the virus by producing antibodies that target the virus precisely. Antibodies bind like a lock-and-key to a specific part of the virus.
The presence of antibodies in the body is often referred to as immunity as these antibodies usually protect against re-infection and return of the same disease. Antibody tests can help scientists fight the pandemic in multiple ways. It can give a more accurate measure of how many people had the new coronavirus (past infections). It would also let health care workers who were ill with COVID-19 symptoms, but were never tested for the disease, return to work – as they maybe now immune to the disease.
However, it is as yet unknown how much protection these antibodies might provide against another infection of COVID-19.
Antibody tests are also critical for assessing population spread of the virus and the level of ‘herd’ immunity in the population. Assessing population spread is important for deciding whether to lift or enforce measures to control the virus (such as quarantine, social distancing, school, and workplace closures).
Antibody tests are very different to RT-PCR – in that it detects patient’s immune antibody response to the virus rather than detecting the virus itself. There two types of protective antibodies that are produced when the immune system encounters the virus, namely IgM and IgG. About 5 days after infection with a new virus (around the same time as symptoms appear), our immune system produces early `prototype’ antibodies (IgM) with intermediate strength binding to the virus. Then, about 8 – 10 days after infection, IgG antibodies with high binding strength are produced, that enable rapid virus clearance.
These antibodies help fight COVID-9 and remain in the blood for months after the virus and disease is cleared. A major advantage is the ability to see if patients are currently infected or have recovered from COVID-19, even if they have fully recovered and cleared the virus a few months back. The main disadvantage is that these tests cannot distinguish between an active and a previous infection. It can only determine if a patient has at some point been infected with COVID-19. A negative result in the antibody test indicates the absence of antibodies against the SARS-CoV-2 virus. However, it could also mean that it is too early in the infection to be positive. For COVID-19 testing, these antibody tests are not as well established – though now many companies are working hard towards producing and testing them in patients.
There are two main types of antibody test that can be used to test for COVID-19. One method is the Enzyme-Linked Immunosorbent Assay (ELISA) and the other is Lateral Flow Assays (LFA). Both assays (tests) require a sample of blood from the patient for testing, as opposed to a sample from a throat or nose swab that is needed for RT-PCR.
Enzyme-Linked Immunosorbent Assay (ELISA) is a widely used biochemical technique that can be used to detect antibodies against an invading virus. ELISAs need to be performed in a laboratory by trained technicians. The ELISA test provides important information for diagnosis, management and recovery from COVID-19 and will also help researchers evaluate how many people in the population have been infected, which is important for planning infection control.
The test uses enzymes which are linked to antibodies that can attach to the specific viral protein molecule (antigen) that is being tested for. The enzymes produce a colour change that can be measured by a special machine. The intensity of the colour change gives scientists an idea of the number of molecules of the IgG and/or IgM antibodies in the patient sample. ELISAs can be done as standard batches of up to 96 assays completed at the same time, allowing cheap and time effective method for batch testing of large numbers of patient samples at the same time, as well as help speed up the number of patients that can be tested for SARS-CoV-2.
Lateral Flow Assays have commonly been referred to as ‘rapid antibody test’ in the media and are like a pregnancy test. It detects antibodies to the virus from a drop of a patient’s blood, indicating that the patient has COVID-19 or has recovered from COVID-19. LFAs can be completed very quickly per individual (within 15 minutes), are point-of-care tests and can be produced cheaply. The procedure requires very little training to perform and does not rely on specialist laboratories or scientists to analyse.
Ideally, a combination of all the above testing methods should be used to control the COVID19 pandemic. Ultimately, scientific knowledge and research leading to a vaccine or cure could be the saviour of this dreaded disease that has shaken the very edifice of life as we know it. Science could pave the way to a COVID-19 free world, and maybe we shall awake once more every morning with renewed strength and hope.
(The writer, Dr. Nafeesa Noordeen is a medical research scientist in molecular biology and biochemistry, trained at Imperial College of Science Technology and Medicine and UCL, UK. She also teaches a Master of Science course in Molecular Pathology at the Human Genetics Unit, Medical Faculty, University of Colombo.)


