News
Wonder drugs vs super-bugs: The battle is on

Dr. Lakmini Wijesooriya
It was a ‘medical miracle’ which opened up amazing frontiers. The discovery of ‘mould’ which could kill off bacteria way back in 1928, by chance, and the subsequent development of penicillin launched the ‘modern antibiotic era’.
Antibiotics cure numerous diseases and pull back millions of men, women and children from the brink of death.
Have we come full circle, 12 years short of a century after the discovery of the wonder-drugs that are antibiotics? Will we be overcome by super-bugs which have armed themselves against the onslaught of antibiotics and descend into an era ravaged by infectious diseases and mass-scale death?
“Denna deyak nethi vei — we may have nothing to give,” is the plaintive cry being echoed at different levels by numerous health professionals as red-lights signal antimicrobial resistance in Sri Lanka.
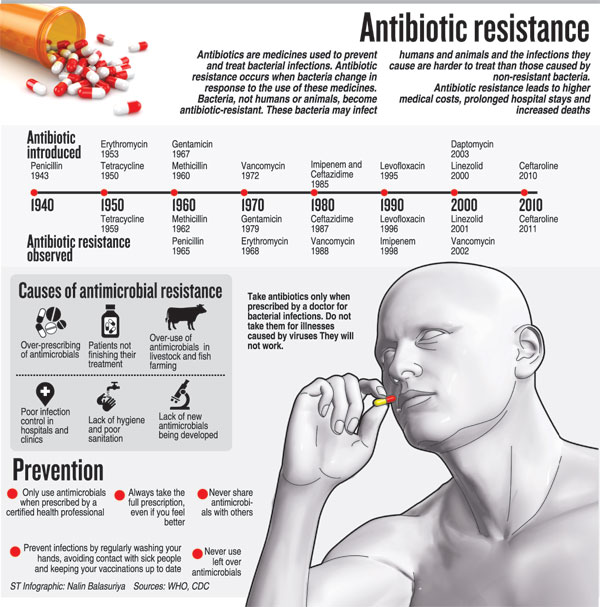
This is as Sri Lanka and the world steps into ‘Antibiotic Awareness Week’ declared by the World Health Organisation (WHO) to spread the message about global antibiotic resistance and encourage best practices among people, health workers and policy-makers to prevent the further emergence and spread of antibiotic resistance.
With the theme being ‘Antibiotics: Handle with care’, the basic but all-important message is that antibiotics (also called antimicrobial agents) are a precious resource and should be preserved. They should be used to treat bacterial infections only when prescribed by a certified human or animal health professional. Antibiotics should never be shared or saved for the future, the Sunday Times learns.
Pointing out that Asia is only second to Africa in its dubious reputation for deaths due to antibiotic resistance, Professor in Medicine of the Ragama Medical Faculty, Kelaniya University, Prof. Ranjan Premaratna reiterates that antimicrobial resistance is due to poor modes of practice.
“It is rushing out of control and we have to take measures to halt and reverse this trend,” he says, going back to the late 1960s when the world thought, and rightly so at that time, that humankind had triumphed over infectious diseases due to antibiotics and vaccines.
However, he warned, now the world is facing huge challenges from new and re-emerging infectious diseases, aggravated by the spectre of drug-resistant diseases such as multi-drug resistant (MDR) tuberculosis (TB), extensively-drug resistant (XDR) TB and MDR P. falciparum malaria. Re-emerging infectious diseases, thought to be controlled through antibiotics, are now resistant to antibiotics.
Prof. Premaratna speaks with evidence in hand through a study on antibiotic sensitivity patterns of 52 patients with ESBL-UTI (extended-spectrum B-Lactamase producing-urinary tract infections) published this year: 44 patients had E-coli bacterial infections and 8 patients Klebsiella bacterial infections. More than half (53.8%) of the patients had no records of ‘recent’ hospitalisation suggesting the high existence of community-acquired ESBL. Nearly all the patients (96.2%) were sensitive to Meropenem, a powerful ultra-broad spectrum injectable antibiotic. 3.8% with E-coli were resistant to Meropenem and had been on multiple antibiotics including Meropenem in the recent past for recurrent UTIs.
He highlights the reasons for multi-drug resistant organisms: Antibiotic misuse, substandard antibiotics, non-adherence to guidelines and wide use of antibiotics in animal husbandry Studies have categorically shown us that there is 96% antibiotic use in the community for viral fever, he says, stressing that antibiotics should only be used for bacterial infections and not for viral infections which have to run their course.
Quoting a study by D.N. Magana-Arachchi et al published in 2010, he says that 3% of 131 TB cultures from the Welisara Chest Hospital were multi-drug resistant.
The world including Sri Lanka seems to be heading towards a ‘black era’ without antibiotics to treat serious illnesses and the dominance of super-bugs, he says, adding, “We’ll be at the mercy of these super-bugs.”
It is Consultant Microbiologist and Senior Lecturer Dr. Lakmini Wijesooriya of the Department of Medical Microbiology, Ragama Medical Faculty, who puts antibiotic resistance under the microscope and takes a long hard look not only at how it is occurring but also how it can be battled.

Prof. Ranjan Premaratna
The common causes for the failure of antibiotic therapy could be – drugs, pathogens or the laboratory. Under drugs, the fault could lie in an inappropriate drug being prescribed; an inadequate dose; improper route of administration; accelerated inactivation and poor penetration, as also poor-quality drugs. Under pathogens there could be drug resistance, a super-infection or a dual infection initially. A laboratory, meanwhile, could also contribute to this situation by issuing an erroneous report.
Referring to the ‘rational’ use of a drug, she points out that it requires the patient to receive medications appropriate to his/her clinical needs, in doses that meet the individual requirements for an adequate period of time and at the lowest cost.
What clinicians should ask themselves before prescribing an antibiotic to a patient is:
Is an antibiotic necessary? This should be after considering the fact that antibiotics are useful only in the treatment of bacterial infections; not all fevers are due to an infection; not all infections are due to bacteria; and there is no evidence that antibiotics prevent secondary bacterial infection in a viral infection.
If an antibiotic is needed, then what is the most appropriate choice of the antimicrobial agent, what dose, frequency, route and duration and how best can the treatment be improved to make it most effective? The ‘choice’ should be based on the three main factors of — Aetiological agent; patient-related factors; and antibiotic-related factors.
“Be careful of the identification of the aetiological agent by the laboratory,” says Dr. Wijesooriya citing the example of a urinary tract infection (UTI). The doctor needs to consider how the urine sample was collected, as contamination is frequent even in the best of conditions. This is why the doctor should also take into account the symptoms, the urinalysis and the most probable agents based on the epidemiology of the resistance-pattern of the bug (causative micro-organism) and clinical experience.
Patient-related factors which need to be considered include age (whether a baby, a child, an adult or an older person), is the patient immuno-compromised, is there renal (kidney) or hepatic (liver) dysfunction, genetic factors and is the patient pregnant or breastfeeding.
Antibiotic-related factors would include the spectrum of organism-coverage by the specific antibiotic, profile of the antibiotic (absorption, excretion, tissue levels, peak levels, etc), toxicity and other adverse effects, drug-drug interactions and cost. An important consideration will be the effective and shortest duration.
With regard to local antibiotic-resistance data, the patterns would vary from hospital-to-hospital and from unit-to-unit even in the same hospital and over time, she adds.
Dangers of irrational antibiotic use  Dr. Poonam Khetrapal Singh Shocking is the image painted by Consultant Microbiologist Dr. Lakmini Wijesooriya with regard to ‘irrational’ antibiotic use worldwide. >50% of all medicines are prescribed, dispensed or sold inappropriately, ½ of all patients fail to take their medicines correctly >50% of all countries do not implement basic policies to promote rational use of medicines In developing countries, less than 40% of patients in the public sector and 30% in the private sector are treated according to clinical guidelines. According to data collected by the WHO in the Southeast Asia region including Sri Lanka: 50% of viral upper respiratory tract infections are treated unnecessarily with antibiotics. Only 53% of pneumonia cases receive an appropriate antibiotic, 54% of acute diarrhoea cases are treated unnecessarily with antibiotics, 40% of prescribed antibiotics are prescribed on an under-dose. Both Dr. Wijesooriya and Prof. Premaratna underscore the need to introduce multidisciplinary interventions to reduce the inappropriate use of antibiotics to increase the lifespan of precious antibiotics. Dr. Wijesooriya picks up the contributory factors for such inappropriate use when she says that there may be a lack of updated provider knowledge. This is while prescriber habits (guidelines versus habit) also come into play, along with poor availability information such as clinical guidelines, lack of continuing medical education and supervision in prescribing. This situation, according to this Microbiologist, is aggravated by excessive pharmaceutical promotions which emphasise the use of the medicines but underplay the negative consequences. She laments that the situation is made worse by very short consultation times (one minute) inadequate for a proper diagnosis, fear of peer-pressure if they are seen prescribing differently to their colleagues and very short patient-dispenser interaction time (seconds) during which there is no proper explanation to the patient on how to take the medicines. Pointing out that the consequences of irrational antibiotic use are long-lasting illnesses, prolonged treatment, more doctor visits, extended hospital stays and increased cost of treatment, Dr. Wijesooriya stresses that the consequences of inappropriate antibiotic use include antimicrobial resistance, adverse drug reactions and medication errors, poor patient outcomes, eroded patient confidence, wastage of resources and increased health-care costs. Focusing on how the misuse of antimicrobials contributes to antibiotic resistance worldwide, she gives the following stark examples: Tuberculosis (TB) – 0-17% primary multi-drug resistance Gonorrhoea – 5-98% penicillin resistance in N. gonorrhoeae Pneumonia and bacterial meningitis – 0-70% penicillin resistance in S. pneumoniae Diarrhoea shigellosis – 10-90% ampicillin resistance and 5-95% cotrimoxazole resistance Hospital infections – 0-70% S. aureus resistance to all penicillins & cephalosporins Unnecessary use or the wrong choice of an antibiotic agent have been shown to be prevalent in both inpatient and outpatient settings |
| Crucial links in war against AMR Antimicrobial resistance (AMR), if present trends continue, is projected to kill 10 million people annually by 2050, while already it is leading to around 700,000 deaths each year, said WHO’s Regional Director for the Southeast Asian Region, Dr. Poonam Khetrapal Singh, pointing out that this threat cannot be contained without close cooperation among the human health, animal health and environmental health sectors. “While establishing this cooperation is of vital concern within countries, it is doubly so at the international level given the cross-border nature of AMR and ongoing concerns regarding emerging zoonotic diseases,” she told a workshop this week in New Delhi, India which focused on resistance emanating from antibiotic use in food animals. “Therefore,I would like to focus on the critical importance of the ‘One Health’ approach and of operationalising the tripartite collaboration between the WHO and the Food and Agriculture Organisation of the United Nations and the World Organisation for Animal Health.” |
| Hand-washing & ‘antibiotic stewardship’ With no major new types of antibiotics being developed in the last 30 years, Microbiologist Dr. Lakmini Wijesooriya reiterates that hand-washing should be given priority in infection-control to eliminate the need for antibiotics in the first place, while also developing and using of vaccines and other alternatives. Did you know that doctors in the United Kingdom are discarding their coats and ties when doing ward rounds because there is a belief that these could be modes of transmission for infections from one patient to another, she asks. She strongly urges ‘antibiotic stewardship’ in all health centres. This means a system of informatics, data collection, personnel and policy/procedures which promotes the optimal selection, dosing and duration of therapy for antimicrobial agents throughout the course of their use. “Such stewardship will limit inappropriate and excessive antibiotic use and improve and optimize therapy and clinical outcomes for the individual infected patient,” she adds. Dr. Wijesooriya resorts to an oft-quoted slogan: Antibiotics save lives, so let’s save antibiotics. |