News
SL hooked on substandard Indian drugs from blacklisted manufacturers
Some of the Pharmaceuticals imported into the country, mostly from India, are repeatedly failing to comply with quality standards, and authorities are facing difficulties in withdrawing them from the outlets after the quality tests are done. According to the Cosmetics, Devices and Drugs Regulatory Authority (CDDRA) of 2012, over 110 drugs have failed to comply with the quality standards specified, while this number has reached 54 in 2013.
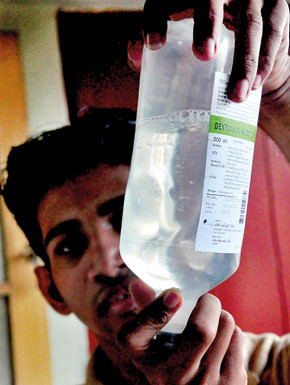
Contaminated saline.Pix by Mangala Weerasekera
In January alone, 13 varieties have been recalled from the market and government sector, due to quality failures. Although circulars are issued islandwide, to recall all items in the batch that fail quality tests, the government cadre is not sufficient to ensure that recall orders are carried out, while there is no proper system in place to ensure the welfare of the patients who have been treated with such drugs.
Pharmaceutical industry on the other hand also calls out on lack of good practices of the government in transporting and storing of pharmaceutical drugs as one of the reasons for quality failures. A majority of the drug varieties that have failed the quality tests are antibiotics while some critical life saving drugs such as Benzylpenicillin for injections, Sodium Valporate and Oral amoxicillin co-amoxyclav:paediatric preparations have also failed in quality.
Almost 90% of the drugs that have failed the quality test are manufactured in India, with a majority of them supplied to the Government via tenders. Further, there are instances where products supplied by a pharmaceutical company have failed the quality test a multiple times, some as high as seven or eight times. Added to this, products supplied by three of the six pharmaceutical companies which were blacklisted in 2011, have failed the quality tests as well.
“We import over 80% of our pharmaceutical drugs from India. However, it is common knowledge that India has substandard drug manufacturers. Sometimes, reputed companies sub contract to these substandard manufacturers, when they do not have the capacity to fulfill their orders as required. We have started a process to ensure this does not happen. We hope the situation will improve with time,” CDDRA Director Dr. H. Beranagama told the Sunday Times.
Speaking on conditions of anonymity, a professional in the local pharmaceutical industry explained that, while the percentage of quality failures may be low, and sometimes unavoidable even in best case scenarios, what is of concern is the multiple failures recorded by the same number of companies. “India now supplies drugs to many countries worldwide. It is not a bad supplier, but most of the companies which supply under government tenders are not even in the top hundred of the pharmaceutical companies. That is where the concern is. However, if we are to go for better quality, the cost of such drugs will also increase substantially,” he explained.
According to Dr Beneragama, with regard to multiple quality failures, he said that Central Drug and Pharmaceuticals and Apurva Biopharm Inc have been blacklisted, but no action has been taken against the other companies.
“When we conducted Good Manufacturing Practices inspections on Apurva Pharmacies, we found that there was no actual manufacturing company, thus we immediately blacklisted the company. Under the existing system, we can only withdraw registration of a company if there has been five quality failures of the same product” he said, adding that, CDDRA is in the process of establishing systems to curtail such instances from future occurrence.
When asked how companies blacklisted in 2011 are still supplying drugs, of which, there have been quality failures again, he explained that the ban was lifted after the CDDRA was satisfied of their manufacturing practices consequent to visits to their plants. However, All Ceylon Government Medical Officers Association (GMOA) President Dr Gishantha Dasanayake states that the ban was lifted as a result of continuous pressure on the Sri Lanka Government by Indian authorities.

Dr Dasanayake alleged that the CDDRA does not have proper quality assurance systems in place for pharmaceutical registrations.
“In ideal situations, when a bulk tender is called, the manufacturer should submit a quality certificate, which should tally with the CDDRA’s pre-assessment test. They should also do random tests on market supplies, which should tally with the earlier reports. This type of rigorous process is not followed by the CDDRA. They don’t have the capacity to do so either”, he said, explaining that the CDDRA does not have the required pharmacists, chemists nor a proper lab to carry out the necessary testing.
Denying allegations that the CDDRA does not have a proper quality assurance process when drugs are registered in the country, Dr. Beneragama said that the process has good checks and balances to ensure the quality of the drugs released to the market.
However, when asked, he admitted that not all drugs go through quality tests before registration, explaining that quality testing is mandatory only for certain types of drugs such as lifesaving drugs, slow release drugs, narrow therapeutic drugs and hormone replacement drugs.
“We don’t have a 100% capacity to assure quality. Although we have a big responsibility, for certain things, we rely on the manufacturer. In these instances, we rely on the quality test certificates the supplier submits to us, which should be from an accredited independent lab,” he said.
At present, there are only five Food and Drug inspectors at CDDRA, while two positions remain vacant. According to Dr. Beneragama, the cadre required by the CDDRA to function effectively is 20 Food and Drug inspectors, while at district level, the number should increase from 48 to 100. “We need at least 50 personnel, if we are to carry out post marketing surveillance properly, and I have sought ministry approval to recruit the required cadre” he added.
Explaining the process in recalling quality failed drugs, Dr Beneragama said that they utilise their provincial and regional officers, health inspectors and medical officers.
However, there is no proper procedure in place to take remedial action on patients who have been administered substandard drugs. “If there are any immediate repercussions or adverse reactions to the said drugs, then we take immediate action, but we don’t have a system beyond that” he said. He also said that, as quality failures vary in their severity, if there is no immediate reaction, the side effects of such substandard drugs may not be that serious.
GMOA Assistant Secretary, Dr Nalin Ariyarathne also confirmed the absence of a system to inform the doctors of quality failures of prescribed drugs, and treat patients without taking such matters into consideration.
“At the end, it is the patient who suffers. There are critical times when drugs such as antibiotics are administered and do not produce the standard results due to quality failures, it could cost the patient’s life. So it is important to know of quality failures, and more importantly to prevent it. I believe that every batch that is supplied should be tested for quality, but is not the case at present” he said.
Follow @timesonlinelk
comments powered by Disqus

